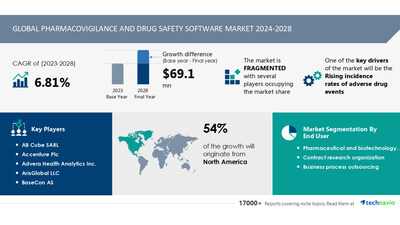
NEW YORK , Oct. 21, 2024 /PRNewswire/ — Report with the AI impact on market trends – The Global Pharmacovigilance and Drug Safety Software Market size is estimated to grow by USD 69.1 million from 2024-2028, according to Technavio. The market is estimated to grow at a CAGR of almost 6.81% during the forecast period. Rising incidence rates of adverse drug events is driving market growth, with a trend towards integration of AI in pharmacovigilance. However, high cost of ownership, installation, and maintenance poses a challenge -Key market players include AB Cube SARL, Accenture Plc, Advera Health Analytics Inc., ArisGlobal LLC, BaseCon AS, Clarivate PLC, Cognizant Technology Solutions Corp., Ennov SAS, EXTEDO GmbH, Honeywell International Inc., Indegene Pvt. Ltd., IQVIA Holdings Inc., Max Application Srl, Oracle Corp., Pegasystems Inc., Sarjen Systems Pvt. Ltd., United BioSource LLC, Veeva Systems Inc., and Wipro Ltd..
AI-Powered Market Evolution Insights. Our comprehensive market report ready with the latest trends, growth opportunities, and strategic analysis- View your snapshot now
Forecast period
2024-2028
Base Year
2023
Historic Data
2018 – 2022
Segment Covered
End-user (Pharmaceutical and biotechnology
companies, Contract research organization, and
Business process outsourcing) and Geography
(North America, Europe, Asia, and Rest of World
(ROW))
Region Covered
North America, Europe, Asia, and Rest of World
(ROW)
Key companies profiled
AB Cube SARL, Accenture Plc, Advera Health
Analytics Inc., ArisGlobal LLC, BaseCon AS,
Clarivate PLC, Cognizant Technology Solutions
Corp., Ennov SAS, EXTEDO GmbH, Honeywell
International Inc., Indegene Pvt. Ltd., IQVIA
Holdings Inc., Max Application Srl, Oracle Corp.,
Pegasystems Inc., Sarjen Systems Pvt. Ltd., United
BioSource LLC, Veeva Systems Inc., and Wipro
Ltd.
Key Market Trends Fueling Growth
Pharmaceutical and life sciences companies are experiencing a significant increase in the volume of pharmacovigilance data generated from various non-standardized sources, leading to complexities in managing legacy technology systems. To address these challenges, pharmacovigilance and drug safety software developers have integrated Artificial Intelligence (AI) technologies into their offerings. This innovation enables advanced technology benefits, such as machine learning and natural language processing , to solve industry-specific problems using cognitive automation. AI-based software solutions, like LifeSphere Safety by ArisGlobal and Intelligent Safety Suite by Indegene, offer advanced analytical capabilities, real-time virtualization, automatic data storage, and maintenance. The pharmaceutical sector’s growing adoption of AI for predictive analytics, drug discovery, and big data tools has created a favorable ecosystem for AI-integrated pharmacovigilance and drug safety software. This trend is expected to bring a paradigm shift by enhancing data quality, reducing human resources, and handling large data volumes. The global market for pharmacovigilance and drug safety software is poised for growth due to these factors.
Pharmacovigilance and drug safety software market is witnessing significant growth due to the increasing need for effective drug safety monitoring. Expensive technology, such as issue tracking software and advanced technologies like cloud-based delivery mode, are being adopted by biotechnology companies, hospitals, and healthcare providers to enhance drug safety. Pharmacovigilance services play a crucial role in public safety by detecting, assessing, and preventing adverse drug reactions (ADRs) and severe adverse events. The market is driven by the globalization of pharmacovigilance, trial and post-marketing monitoring, and the need for real-time reporting of adverse events. VigiBase, a leading pharmacovigilance database, is used for ADR detection and risk evaluation. Medical process improvements, consumer safety, prescriber education, and drug efficacy are key focus areas. Software upgrades and analytical preference towards artificial intelligence (AI) are trends in the market. AI helps in medical monitoring, safety reporting, and drug interaction detection. The healthcare system benefits from timely ADR reporting and compliance with regulatory norms.
Insights on how AI is driving innovation, efficiency, and market growth- Request Sample!
Market Challenges
Pharmacovigilance and drug safety software market involves three main cost categories for both on-premises and cloud-based solutions: initial capital cost, deployment cost, and maintenance cost. Small-scale businesses face high adoption barriers due to the significant costs. On-premises solutions have higher ownership costs, including hardware, network, facility, and security, which are 45-75% more than cloud-based solutions in terms of ongoing personnel costs. Ongoing costs for Software as a Service (SaaS) can account for 80-90% of the total cost. Customized solutions and low-cost offerings from software providers help companies adopt these systems despite the costs, but this may hinder market growth for small-scale pharmaceutical manufacturers who rely less on large volumes of drug safety solutions. Pharmacovigilance and drug safety software play a crucial role in monitoring adverse drug reactions (ADRs) and ensuring drug safety. However, the market faces challenges such as expensive technology, the need for issue tracking software, and cloud-based delivery mode. Biotechnology companies, hospitals, and healthcare providers rely on these solutions for drug safety monitoring during both drug development and post-marketing phases. Public safety is paramount, and advanced technologies like AI and machine learning are being used to detect, assess, and prevent severe ADRs. Pharmacovigilance services help evaluate risks, manage medical processes, and report adverse events to regulatory bodies. Consumers, prescribers, and healthcare systems benefit from real-time data availability and reporting norms. Despite these advancements, challenges remain, including software upgrades, disparate data, and analytical preference. VigiBase, a leading pharmacovigilance database, plays a critical role in ADR detection and prevention. However, addressing issues like drug interactions, medical monitoring, safety reporting, and adverse drug reactions remains essential. The trial and post-marketing phases require continuous monitoring, and globalization of pharmacovigilance adds complexity. INTIENT Pharmacovigilance and other providers help navigate these challenges, ensuring drug efficacy, managing side effects, and maintaining drug safety. Insights into how AI is reshaping industries and driving growth- Download a Sample Report
Segment Overview
This pharmacovigilance and drug safety software market report extensively covers market segmentation by
End-user 1.1 Pharmaceutical and biotechnology companies 1.2 Contract research organization 1.3 Business process outsourcing Geography 2.1 North America 2.2 Europe 2.3 Asia 2.4 Rest of World (ROW) 1.1 Pharmaceutical and biotechnology companies- Pharmacovigilance and drug safety software plays a crucial role in ensuring patient and product safety for pharmaceutical and biotechnology companies. This software is used for pre-market and post-market monitoring, identifying unrecognized adverse drug reactions and events, quantifying risk, safety labeling , and refuting false safety signals. The global market for pharmacovigilance and drug safety software is growing due to stringent regulatory requirements from authorities like the US FDA, EMA, CFDA, and JFDA, as well as the increasing number of clinical trials. Major markets include the US, Japan , Canada , and India , driven by high pharmaceutical research and development spending and the need for first-in-class drugs. The rising demand for pre-market drug safety due to clinical trials for chronic diseases and high drug consumption is also fueling market growth. Pharmaceutical companies focus on vigorous pharmacovigilance and drug safety monitoring to bring new molecular entities and biosimilars to market.
Download complimentary Sample Report to gain insights into AI’s impact on market dynamics, emerging trends, and future opportunities- including forecast (2024-2028) and historic data (2018 – 2022)
Research Analysis
Pharmacovigilance and Drug Safety Software is a critical solution for healthcare systems, biotechnology companies, hospitals, and healthcare providers to ensure drug safety and monitor adverse events. With the increasing availability of data from various sources, the need for advanced software to process and gain insights from this data is paramount. The software helps in collecting, managing, and reporting adverse drug reactions (ADRs) and drug interactions in compliance with regulatory reporting norms. The software upgrades continually to incorporate the latest analytical preferences and advanced technologies such as Artificial Intelligence (AI) to identify trends and patterns in ADRs. The cloud-based delivery mode ensures easy access to real-time data and enables monitoring of ADRs from anywhere. Disparate data from various sources, including clinical trials, healthcare providers, and regulatory bodies, can be integrated and analyzed to gain comprehensive insights. The software also helps in monitoring drug safety during drug development and post-marketing phases, ensuring public safety. The software addresses compliance issues by ensuring adherence to regulatory reporting norms and providing alerts for potential safety concerns. It also facilitates medical monitoring and enables healthcare providers to make informed decisions regarding patient care. Overall, Pharmacovigilance and Drug Safety Software is an essential tool for ensuring the safety and efficacy of drugs in the healthcare system.
Market Research Overview
Pharmacovigilance and Drug Safety Software is a critical tool for healthcare systems and pharmaceutical companies to ensure the safety of drugs in the market. With the increasing availability of data from various sources, reporting norms have become more stringent, necessitating advanced software solutions. These systems help in monitoring adverse events, drug interactions, and adverse drug reactions (ADRs) in real-time. Disparate data from various sources, including clinical trials and post-marketing surveillance, can be integrated using analytical preference and AI technologies. The software upgrades are essential to keep up with the latest reporting norms and regulatory requirements. INTIENT Pharmacovigilance, VigiBase, and other similar solutions are used for safety reporting, detection, assessment, prevention, and medical monitoring of adverse events. The software also facilitates issue tracking, risk evaluation, and severe adverse event reporting. The globalization of pharmacovigilance and the expensive technology involved make it a challenge for small healthcare providers and hospitals. However, cloud-based delivery modes and advanced technologies, including AI and biotechnology, are making these solutions more accessible. Drug development, drug safety monitoring, public safety, and medical process all benefit from these software solutions, which are essential for consumers, prescribers, and drug manufacturers to ensure drug efficacy, side effects, and risk evaluation.
Table of Contents:
1 Executive Summary
2 Market Landscape
3 Market Sizing
4 Historic Market Size
5 Five Forces Analysis
6 Market Segmentation
End-user Pharmaceutical And Biotechnology Companies Contract Research Organization Business Process Outsourcing Geography North America Europe Asia Rest Of World (ROW)
7 Customer Landscape
8 Geographic Landscape
9 Drivers, Challenges, and Trends
10 Company Landscape
11 Company Analysis
12 Appendix
About Technavio
Technavio is a leading global technology research and advisory company. Their research and analysis focuses on emerging market trends and provides actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions.
With over 500 specialized analysts, Technavio’s report library consists of more than 17,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio’s comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios.
Contacts
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
Email: media@technavio.com
Website: www.technavio.com/
View original content to download multimedia:https://www.prnewswire.com/news-releases/pharmacovigilance-and-drug-safety-software-market-to-grow-by-usd-69-1-million-from-2024-2028–driven-by-rising-adverse-drug-event-rates-with-ai-driving-market-transformation—technavio-302281365.html
SOURCE Technavio









Leave a Reply